Quality & Regulatory
How we do
We adhere to ICH Q7A & ISO 9001 quality standards.
Quality
Our Systems are laid upon guidelines of ICH Q7A & ISO 9001 quality standards.
We have been associating ourselves with innovators and global generic pharma companies over the years. Every interaction with these companies has accelerated our learning process. Regular customer visits/audits – most of them being global pharma companies enabled us improve and keep upgrading our quality systems.
We have been associating ourselves with innovators and global generic pharma companies over the years. Every interaction with these companies has accelerated our learning process. Regular customer visits/audits – most of them being global pharma companies enabled us improve and keep upgrading our quality systems.
Quality Assurance
An independent and autonomous function ensuring that the entire product supply chain works
according to company’s standard operating procedures and compliance with cGMP practices.
Quality Control
Well-equipped laboratories performing comprehensive tests for the finished products, WIP, raw
materials, equipment cleaning in compliance to ICH guidelines using Pharmacopoeial methods, In-house
test methods and customer's requirements.
Quality Control Lab is equipped with:
Quality Control Lab is equipped with:
- HPLCs
- GC Units with Head space for residual solvents
- UV Spectrophotometer
- FTIR Spectrophotometer
- Particle Size analyzer (Malvern)
- Polarimeter
- Walk-in type Stability Chamber
- Potentiometric Titrator
- Karl-Fischer
- Lab equipment (pH meter, conductivity meter, analytical balances)
- Modern Microbiological Lab
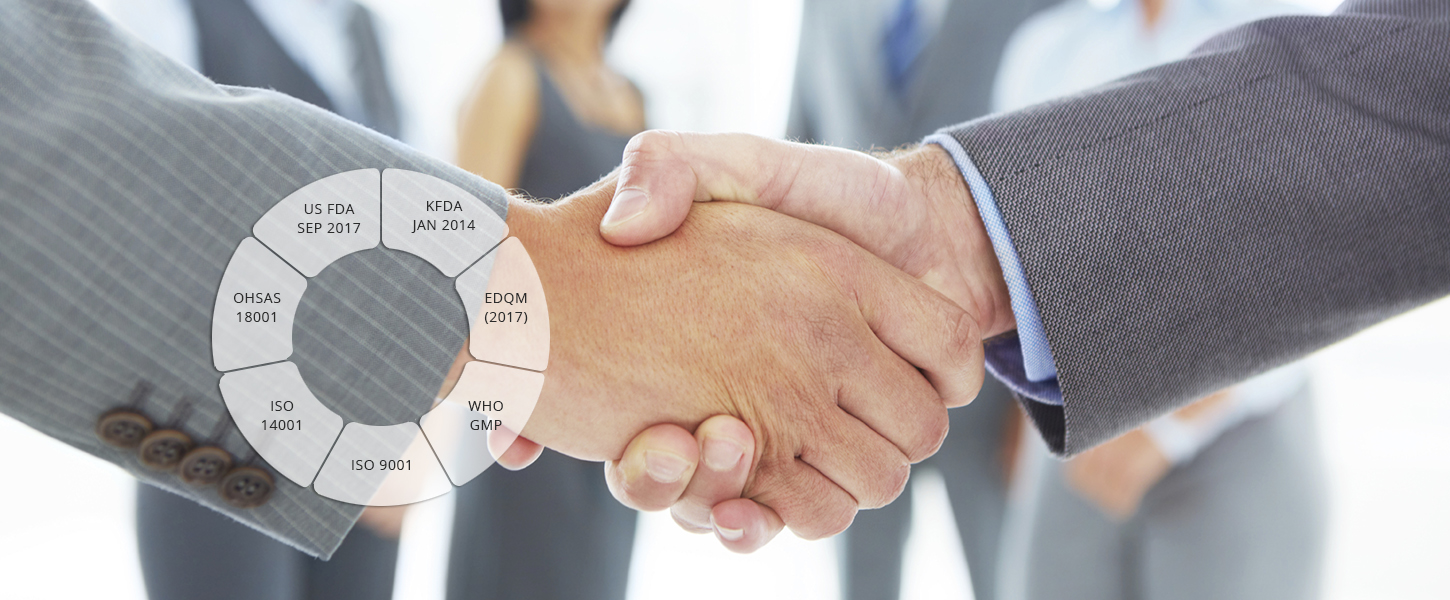
Regulatory Approvals and Filings
- Successfully inspected/approved by US FDA (2010, 2014, 2017), EDQM (2017) KFDA (2014), WHO GMP (2014)
- Japanese Accreditation issued by Ministry of Health, Labour and Welfare (2014)
- On-going DMF/CEP filing activity for various products across the globe
